Month: November 2020
-

Exocrine Pancreas May Play Integral Role in T1D Development
For years, the focus of type 1 diabetes (T1D) research has been on the endocrine pancreas, as that is where the islets of Langerhans reside that secrete insulin, glucagon, and […]
-

Gestational Diabetes Complications Identify Fertile Women at Risk of Permanent Type 1 and Type 2 Diabetes
During pregnancy, women are tested for gestational diabetes mellitus (GDM) to manage their overall health. Gestational diabetes complications can put both women and their babies at risk if not properly […]
-

Kraemer Family Update
Remember Hayden!? His story was featured in our 2019 organizational film, “Connect for a Cure,” which debuted at our 2nd annual Del Mar Dance for Diabetes. In this impactful interview, […]
-

DRC’s 2020 Virtual Events
It is safe to say that 2020 has been unlike any other year. That being said, DRC’s goal for this year has been to find new ways to connect with […]
-
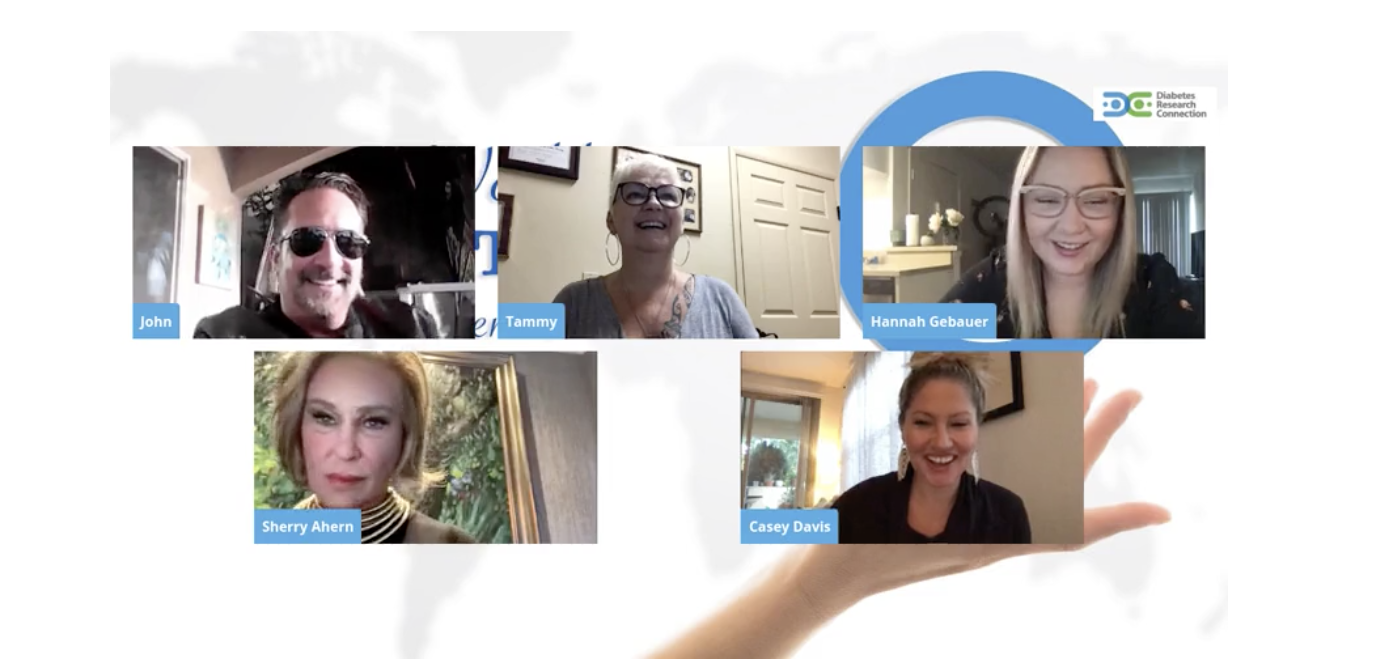
DRC Happy Hour with KSON’s John and Tammy
On Thursday, November 12th, DRC had the opportunity to have a happy hour with John and Tammy, the hosts of KSON, about type one diabetes (T1D) and DRC’s mission in […]
-

Diabetes Awareness Month is Personal for Me
It was 1964 and I was a 15 year old junior in High School, riding home on the subway on a beautiful November day in New York. Looking for something […]
-

DRC’s Co-Founders Interviewed on “Living Better San Diego”
In honor of World Diabetes Day, DRC’s co-founders, David Winkler and Alberto Hayek, MD, were interviewed by Vicki Pepper of “Living Better in San Diego.” This show features Information and […]
