Chris and Lorraine Stiehl have committed to raise funds to name an entire Research Grant titled the Stiehl Legacy Project. Chris is a survivor of type 1 diabetes. At age 10, his physician said he wouldn’t make it to 50. At 70, he has beaten the odds. Why? Because of the advances being made in diabetes research!
Final Project Update
With our resolve to create a platform to maintain the long-term function of the islet grafts and simultaneously protect them from immune rejection, we believe in having made considerable progress toward achieving our aim.
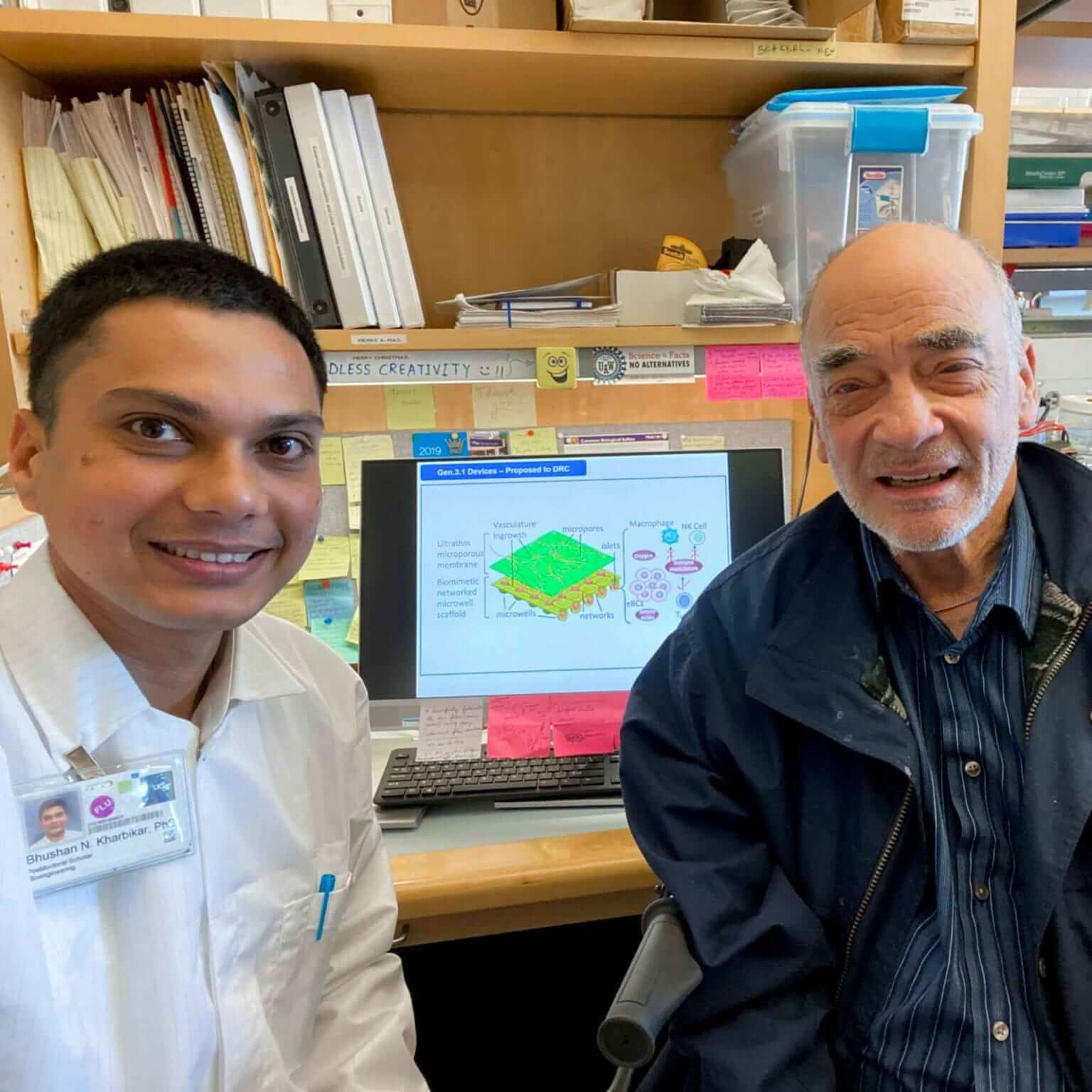
After reporting the successful development of a biomimetic 3D architecture for islets for implantation, which improved their functional performance, we also created local tubular depots with immune modulators to provide the immune privileged environment to the islet graft in the kidney capsule. The depots release the immunosuppressive drug locally to prevent graft rejection.
We have now developed the next generation of these devices by providing islet placements. These new bilaminar circular designs of the device increase the drug holding capacity by ten times. We then worked on the prospective new transplantation strategy under the peritoneal membrane with a newly developed device for local immune modulation. This new strategy will provide a larger space to accommodate our larger bilaminar circular device with arc shape cavity for more islets and ease of access to the vasculature. Hence, our new devices also demonstrated better and long-term controlled and sustained release of the immunomodulatory drug, which is crucial for preventing graft rejection.
We take this opportunity to give huge thanks to DRC and our very generous donors for enabling this research. We hope to keep conveying further technological developments in our quest to find the cure for type 1 Diabetes.
6-Month Project Update
Our group has developed cell encapsulation technology in the recent past and proven its successful application to protect implanted islets from immune attacks. Long-term graft survival and preserving its functional performance for optimal glucose sensing and insulin secretion are still a challenge. Researchers in the field are facing a catch 22 situation with the present generation encapsulation technologies. If we implant the islets freely, they get proper nutrition and function well but are attacked and killed by the body’s immune system. If we want to protect the cells from immune attacks with an encapsulation device, they end up starving to death. We hope to resolve this deadlock with our novel engraftment strategy with the biomimetic design of our bioengineered pancreas. We have made noticeable progress in achieving our objective.
We successfully developed and optimized a biomimetic 3D architecture for hosting islets during implantation. This spatial architecture mimics the islets’ native environment in the natural pancreas. Alone, these spatial constructs with the engineered islets significantly contributed to the long-term survival and maintenance of islets functions. The additional analysis indicated its role in further improvement in the functional efficacy of the implanted islets. We further plan to aid these biomimetic constructs for engineered islets with a magnitude of local enrichments to enhance their capabilities in getting enhanced vasculature, ample nutrition, sufficient oxygen, and adequate protection against immune attack. The supporting delivery system for these enrichments is tested successfully. In the next phase, we use the delivery system to build the local enrichment infrastructure regimen to ensure successful long-term islet implantations.
To know our latest research towards developing the successful treatment for Type 1 Diabetes, please stay tuned. We want to thank and give humungous credits to DRC and our very generous donors who enable this research.
PROJECT DESCRIPTION
Novel engraftment strategies for a bioengineered pancreas in the treatment of Type 1 Diabetes
Beta-cell replacement therapy restores normal glucose homeostasis by replenishing the lost beta cells through transplantation in the treatment of patients suffering from Type 1 Diabetes. Although this therapy can restore normoglycemia without ongoing insulin supplementation in preclinical models, much development and translational work are yet to be done before the full potential of this therapy can be realized.
Some of the most formidable challenges include (a) the acute shortage of donor islets which limit the treatment to only a few patients with the most severe diabetes, (b) the need for lifelong immunosuppression, and (c) graft failures due to deprivation of essential nutrients, ischemia caused by lack of oxygen, and immune-mediated rejection. Researchers are exploring novel strategies to address these challenges, such as using more readily available cell sources (e.g., xenogeneic islets, stem cell-derived beta cells) and developing immunoprotective cell-encapsulation platforms.
While encapsulation devices can be beneficial in diminishing an immune attack, these devices create a formidable barrier for the vasculature to reach beta cells, leading to nutrient starvation and ischemia, which can impair long term physiologic performance and lead to consequential loss of the graft. Patients lose nearly 70-80% of the graft in the first week of implantation.
My research is focused on developing a bioengineered pancreas that incorporates novel engraftment strategies which can (a) promote vascularization around beta cells and minimize ischemic injury, and (b) create a local immune protective zone by increasing immune tolerance for the graft to preserve the beta-cell mass and long-term normal physiologic performance. Renewable sources of beta cells (derived from human stem cells) from our collaborators will be used to address the acute shortage of donor islet.
Our proposed encapsulation strategies present a biomimetic spatial arrangement for transplanted islets and facilitate a more homogenous distribution of nutrients and oxygen while preventing the accumulation of metabolic waste. The approach allows for direct contact with blood vessels by allowing the ingrowth of new vasculature. The local release of essential amino acids and resident oxygen generation further aids the survival and functional maintenance of the graft. The local delivery of immunomodulatory drugs as part of our engraftment strategies will help to mitigate the risk of immune attack by creating a local protective zone around the islet grafts. Thus, our novel engraftment strategies for this bioengineered pancreas ensures long-term graft survival and preserves functional performance.
The anticipated outcome of our research would lead to a significant leap toward clinical translation of the beta cell replacement therapy for patients suffering primarily from autoimmune T1D diabetes.