Final Project Update
Our work on the DRC project has fostered new collaborations between our group and other islet biologists at Indiana University and the University of Chicago, leading to a U01 grant proposal in spring 2022. New joint experiments are ongoing to apply our findings to human T1D and will likely result in more publications and extramural funding by the NIH.
Once again we are grateful for this unique funding opportunity through the Diabetes Research Connection. Your funds bridged a critical period in our lab’s work and enabled discovery-based, patient-oriented research in T1D. We would like to express our heartfelt thanks to the donors and the Diabetes Research Connection administrative team for your vision in supporting our work.
Click HERE to view the full project update.
6-Month Project Update
In the first six months of funding, we successfully used immunohistochemistry methods to identify the primary cilium on murine and human islet cells. These are hair-like organelles that protrude from the surface of endocrine cells that mediate crosstalk among neighboring cells. We found that, in murine and human pancreas, alpha and beta cells are monociliated and appear to express canonical cilia protein markers including Arl13b, Acetylated alpha tubulin, gamma tubulin, and polyglutamylated tubulin. Furthermore, we find that mouse and human islet cilia require different staining techniques, thus complicating our study but was a worthwhile troubleshooting experience.
To investigate loss-of-function effects of cilia in rodent and human islets, we generated both alpha and beta-cell specific cilia deletion models in mice, and targeted knockdown of cilia in human islets through either transient shRNA transfection or lentivirus infection. Pilot studies show excellent knockdown efficiency, up to 50-70% by cilia protein and mRNA expression. We are currently investigating the effects of cilia loss on mature beta cell function, including glucose-stimulated insulin secretion, calcium dynamics, Wnt signaling, and beta cell identity maintenance.
For the next six months, we aim to continue our characterization of mouse and human islet cilia expression but also move into diabetes models. To this end, we have already generated two new mouse lines, crossing the beta cell cilia knockout onto the NOD background to mimic type 1 diabetes. We want to test whether cilia loss changes the propensity with which NOD mice develop type 1 diabetes. As cilia is implicated in mediating cellular crosstalk, potentially even between endocrine and immune cell types, we hypothesize that NOD mice will have reduced immune cell infiltration into their islets and, consequently, decreased incidence of type 1 diabetes. Our preliminary results suggest that indeed NOD-bCKO islets show fewer infiltrating CD3+ immune cells compared to age-matched NOD mice, and they are less hyperglycemic. These studies, upon completion, will give us new information on the role of cilia in mediating autoimmune type 1 diabetes.
We appreciate this unique funding opportunity through the Diabetes Research Connection and will continue to use your funds toward discovery-based work in T1D. Thank you and we look forward to reporting our next six months of progress.
Project Description
Type 1 diabetes (T1D) is a disease of the insulin-producing, pancreatic beta cells. As the beta cells progress from normal, to inflamed, to diabetic, there are many structural changes that occur which lead to impaired insulin secretion. We have determined these transport structures (primary cilium) impact beta-cell function. Our research is designed to characterize how changes in the wave-like motion of primary cilia may contribute to, or cause, the onset of T1D.
Primary cilia are hair-like tiny organs (organelles) that protrude from the surface of most cells. They act as antennae to receive information from the environment or neighboring cells. Each beta cell has a single primary cilium composed of a tiny tube-like structure that transports proteins and provides signals to other molecules. Mutations in cilia genes cause human disease, including childhood-onset obesity and diabetes. Diabetic islets have abnormal cilia gene expression. We do not know whether primary cilia loss causes diabetes, or results from the disease.
We hypothesize primary cilia regulate communications between the beta cells in the pancreatic islet cells. As a first step to address whether cilia cause the destruction of beta cells, we will determine when cilia loss occurs during the progression of T1D and determine if cilia loss accompanies the functional loss of beta-cell insulin secretion.
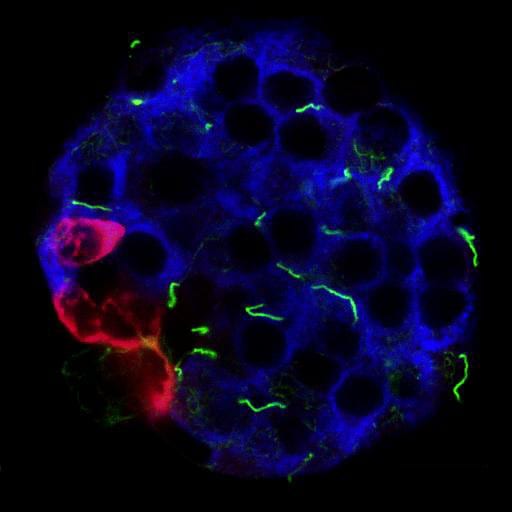
Our research group is a multidisciplinary team of scientists with expertise in islet biology, immunology, and endocrinology. We will examine primary cilia in normal and diabetic islets from T1D animal models. The results are expected to reveal new roles of primary cilia in beta-cell function and endocrine-immune cell crosstalk which may cause the onset of T1D in humans.
As a physician, I see patients with T1D in the clinic and witness the progression of the disease. While the scientific goal of my research is to understand how cilia maintain beta-cell function, my goal is to improve the quality of life for individuals with T1D. I envision our work may lead to the development of cilia-based therapy which will promote beta-cell survival and reduce insulin requirements for those living with T1D, and possibly delay or avoid the onset of the disease.