Final Project Update
I am excited to share our final report. First, I want to thank the DRC and donors for all the support for this project. Our findings give us insights on how glucagon-secreting cells function in response to hypoglycemia, a major limitation for diabetes control. We investigated some important signaling pathways to determine how mTORC1 signaling in pancreatic alpha cells regulates glucagon secretion and mass. We generated two animal models to study this pathway in mice by knocking out important proteins involved in downstream mTORC1 signaling in alpha cells. We were able to identify critical components in the mTORC1 pathway that are crucial to understanding how alpha cells adapt to improve diabetes management and prevent complications.
Project Description
With type 1 diabetes (T1D), glucagon secretion is dysregulated which plays a major role in the resistance to insulin. T1D patients can exhibit failure to secrete glucagon in response to hypoglycemia. This makes the patient more susceptible to develop recurrent hypoglycemia. This project’s focus is to determine how a specific protein helps control glucagon secretion and responses to hypoglycemia. The answers enhance the understanding of how other cells can adapt to states of overnutrition and insulin resistance and positively impact T1D management.
Diabetes mellitus is one of the most prevalent conditions affecting human health in the 21st century. Most of the efforts to understand the pathogenesis and therapy of this disease has focused on two major components: insulin sensitivity and insulin secretion. However, another important component in regulation of glucose homeostasis is glucagon. In physiological states, glucagon plays a major role in maintaining glucose homeostasis by promoting glucose production via hepatic glycogenolysis and gluconeogenesis. In pathological conditions such as type 1 diabetes (T1D), glucagon secretion is dysregulated and plays major roles in the pathogenesis of hyperglycemia.
There are several clinical implications of glucagon biology in T1D:
- hyperglucagonemia induced by the lack of paracrine control from insulin has a major impact in the glycemic volatility, glucose control and increased susceptibility to hypoglycemia, a devastating complication of diabetes.
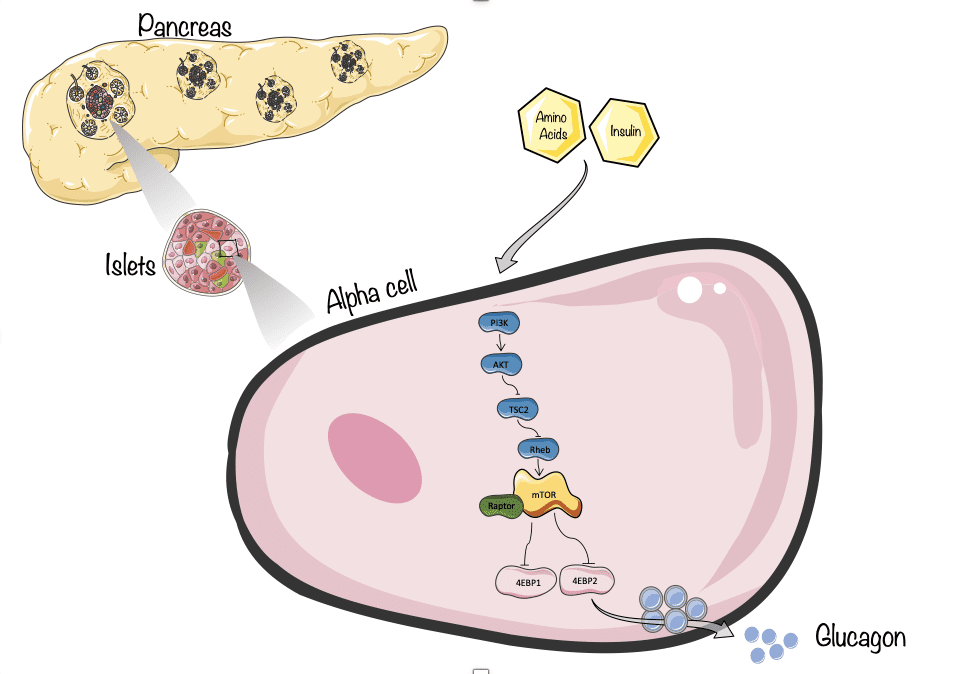
- Significant published evidences support the concept that patients with T1D exhibit failure to secrete glucagon in response to hypoglycemia. This defect in counterregulatory responses to hypoglycemia in T1D makes these patients more susceptible to develop recurrent hypoglycemia. However, the mechanisms responsible for increased glucagon secretion in T1D remain unclear. Published data including our own demonstrates that downstream of insulin and amino acids signaling, the mTOR complex 1 (mTOR/Raptor) is a critical regulator of a-cell mass and glucagon secretion. Preliminary studies demonstrate that genetic activation of mTORC1 by deletion of TSC2 in a cells results in increase in a-cell mass and hyperglucagonemia. This evidence underscores the importance of the insulin/amino acid/mTORC1 signaling on regulation of a-cell mass and function. We identified TSC2/mTORC1 signaling as a major pathway regulating a-cell mass and glucagon secretion. This project focus in determine how mTORC1 acting on key downstream targets control a-cell mass, glucagon secretion and responses to hypoglycemia.
However, uncertainties remain as to (1) How mTORC1 acting on key downstream targets, S6K and 4E-BPs, regulate a cell mass expansion and glucagon secretion (2) what are the individual contributions of S6K and 4E-BPs to regulation of a-cell mass and glucagon secretion, (3) importance of these pathways in human a-cell mass and glucagon secretion. Answers to these questions are critical to understand how a-cells adapt to states of overnutrition and insulin resistance and will impact T1D management.
We hypothesize that mTORC1 regulates a-cell mass and glucagon secretion mainly by a balance between S6K and 4E-BP signaling. To answer these questions, we will: 1. Establish the role of mTORC1/4E-BP/eIF4E axis in regulation of a-cell mass and glucagon secretion. And 2. Identify the importance of mTORC1/S6K on the control of a-cell mass and glucagon secretion.
It is clear that after an “insulinocentric” era, the interest in enhancing our understanding of the mechanisms of glucagon secretion in relation to T1D has increased, with a focus on mainly two research areas:
- To develop novel approaches that suppress the paradoxically high glucagon levels observed in T1D.
- To improve the blunted glucagon response to hypoglycemia.













