Month: September 2020
-
Concerns About Glycemic Control Among Youth and Young Adults with Type 1 Diabetes
Glycemic Control Among Youth with Type 1 Diabetes Scientists have come a long way in their understanding of type 1 diabetes and in not only treatments used to manage the […]
-

Reducing the Need for Systemic Immunosuppression for Islet Grafts
Immunosuppression for Islet Grafts One of the approaches scientists have been testing for reversing or better controlling type 1 diabetes is the use of allogeneic pancreatic islet transplants. By reintroducing […]
-
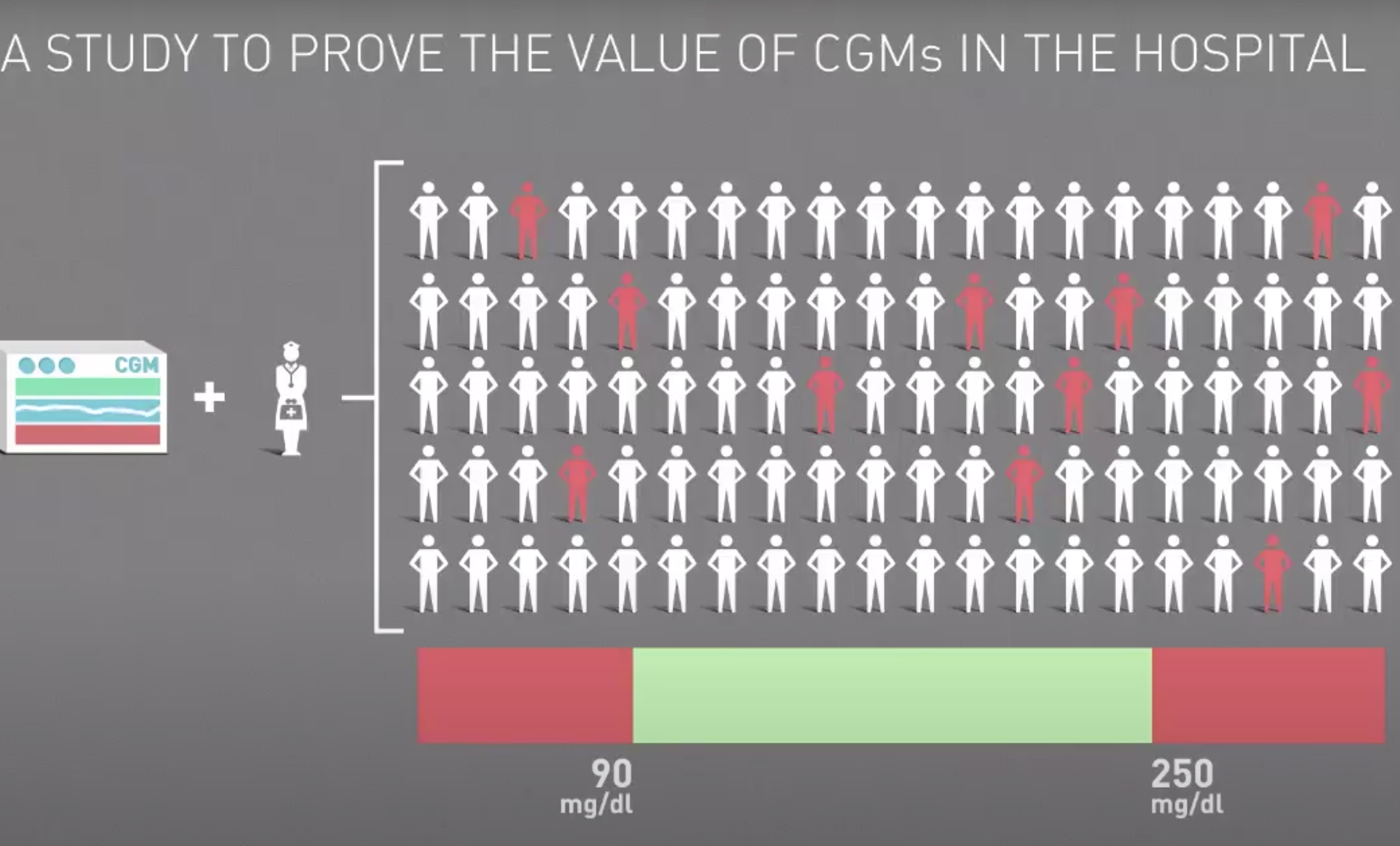
Reducing COVID-19 Deaths and Other Complications for Patients Hospitalized with Diabetes
CGM Use In Hospitals Covid-19 patients who have diabetes experience a higher mortality rate than the general population. A new protocol incorporates the use of continuous glucose monitors (CGM) to […]
-

Advances in Maintaining Beta-Cell Function in Relation to Type 1 Diabetes
Maintaining Beta-Cell Function with T1D In healthy individuals, pancreatic beta cells respond to glucose levels in the blood and automatically increase or decrease the production and release of insulin. This […]
-
Evaluating the Benefits of Continuous Glucose Monitor Use
Benefits of CGM Use Individuals with type 1 diabetes (T1D) have multiple options for managing their blood sugar, ranging from traditional finger sticks and insulin injections to continuous glucose monitors […]
-

New Drug May Delay Onset of Type 1 Diabetes
In many patients, there is a slight delay between the time when type 1 diabetes (T1D) is first diagnosed, and when they become dependent on insulin. This is known as […]
-

Managing Type 1 Diabetes and COVID-19
Type 1 Diabetes and COVID-19 As cases of COVID-19 continue to spread across the United States and the globe, scientists are especially interested in how it affects specific populations, such […]
-
Exploring the High Costs of Diabetes Management
High Costs of Diabetes Discussions around type 1 diabetes care and affordability often focus on the cost of insulin. While insulin prices can be extremely high and add up quickly […]
