Month: September 2016
-

U.S. FDA approves Medtronic’s ‘artificial pancreas’ for diabetes
Original article published by Yahoo! Finance. Click here to read the original article. Medtronic Plc won U.S. approval on Wednesday for an “artificial pancreas” that is the first device to […]
-
Support a Cure for Type 1 Diabetes on Gladitood
Millions of children and adults struggle with type 1 diabetes (T1D). Throughout the month of October, we’ll be raising money to help find a cure on Gladitood, a crowdfunding platform […]
-

An Important Talk About The Importance Of Diabetes Awareness
Original article published by The Huffington Post. Click here to read the original article. National Diabetes Awareness Month is right around the corner, and it brings up the concern regarding […]
-
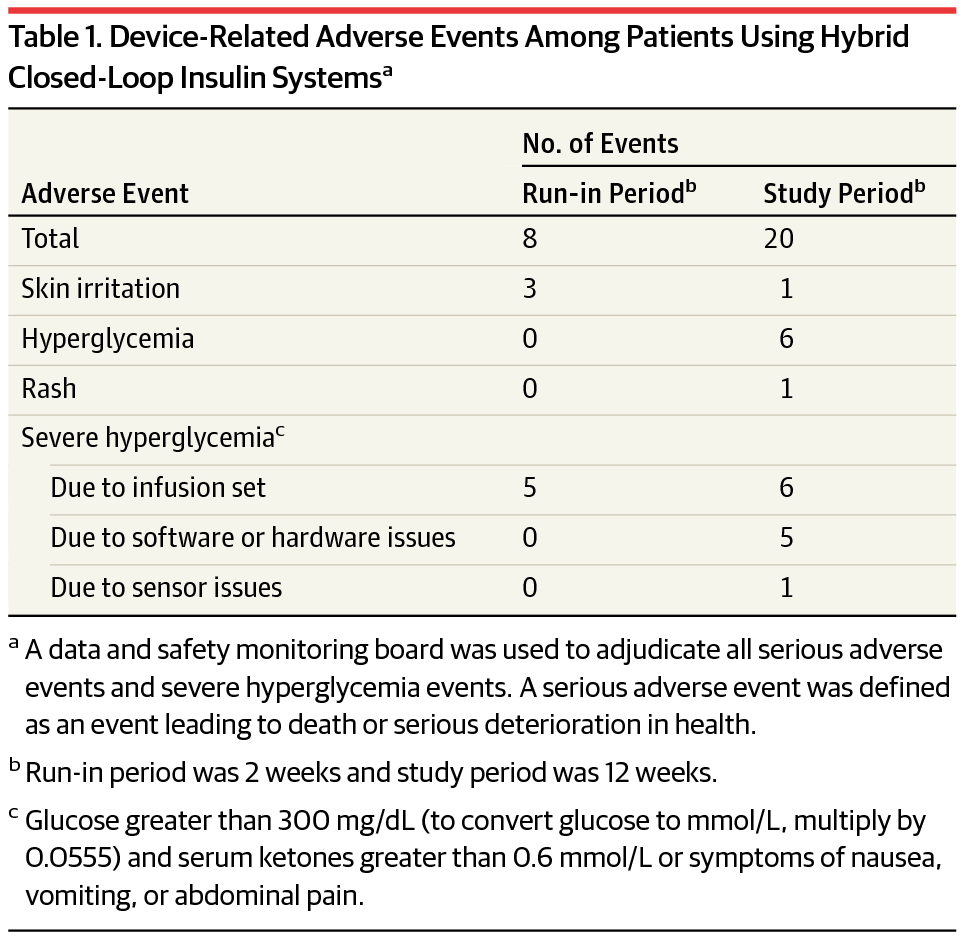
Safety of a Hybrid Closed-Loop Insulin Delivery System in Patients With Type 1 Diabetes
Original article published by The Journal of the American Medical Association. Click here to read the original article. Closed-loop artificial pancreas technology uses a control algorithm to automatically adjust insulin […]
-
Does CGM Benefit Injection Users? Yes! Results from Dexcom’s DIaMonD Study
Original article published by diaTribe. Click here to read the original article. Continuous glucose monitoring (CGM) is often considered a technology for insulin pump users – not those on injections. […]
-

Type 1 Diabetes and Diabetic Alert Dogs
Dogs are often called a man’s best friend – but for some, this common phrase has a much deeper meaning. Groups like Canine Hope for Diabetics and Diabetic Alert Dogs […]
-
![What Are The Types of Diabetes? [INFOGRAPHIC]](https://diabetesresearchconnection.org/wp-content/uploads/Screen-Shot-2016-09-20-at-2.28.35-PM.png)
What Are The Types of Diabetes? [INFOGRAPHIC]
The term “diabetes” refers to a group of diseases that result in problems with blood sugar levels. Each type of diabetes has a different root cause. View the infographic below […]
