Original article published by The Journal of the American Medical Association. Click here to read the original article.
Closed-loop artificial pancreas technology uses a control algorithm to automatically adjust insulin delivery based on subcutaneous sensor data to improve diabetes management. Currently available systems stop insulin in response to existing or predicted low sensor glucose values, whereas hybrid closed-loop systems combine user-delivered premeal boluses with automatic interprandial insulin delivery. This study investigated the safety of a hybrid closed-loop system in patients with type 1 diabetes.
Methods
Patients aged 14 to 75 years with type 1 diabetes for at least 2 years, glycated hemoglobin (HbA1c) less than 10%, and more than 6 months of insulin pump use were recruited from 10 centers (9 in the United States, 1 in Israel) between June 2, 2015, and November 11, 2015. This before and after study had a 2-week run-in period (baseline) for patients to learn the devices without the automated features followed by a 3-month study period with the initial 6 days used to collect insulin and sensor glucose data for the hybrid closed-loop algorithm. In the study period, there was a 6-day hotel stay during which 1 day was used for frequent sampling of venous blood glucose to verify the accuracy of the system. The last patient visit was March 7, 2016. Two central and 4 local institutional review boards approved the study. Written informed consent was obtained from adults and parents, and written assent from minors.
The system included investigational continuous glucose monitoring sensors with transmitters, insulin pumps displaying real-time glucose data, a proprietary algorithm, and blood glucose meters. Patients were required to periodically calibrate sensors and enter carbohydrate estimates for meal boluses. Every midnight, multiple parameters were automatically adjusted by the algorithm.
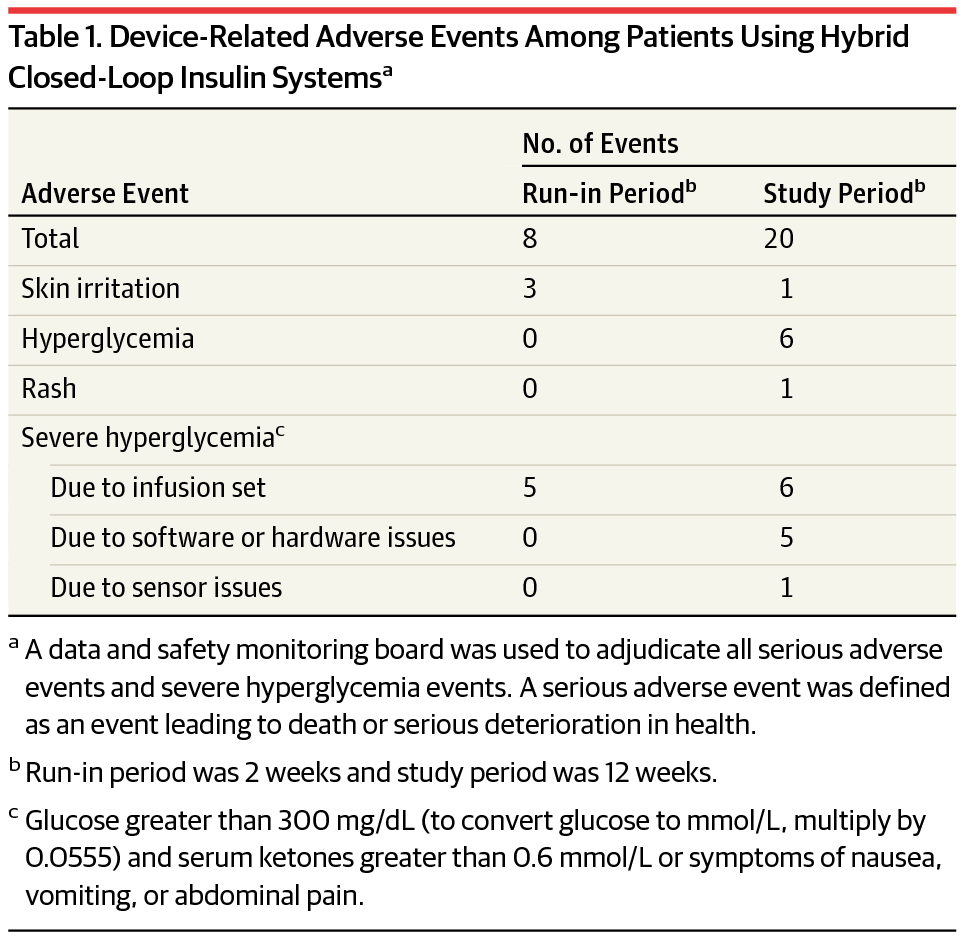
Safety end points obtained during the run-in and study periods (including the hotel stay) were the incidence of severe hypoglycemia and diabetic ketoacidosis, serious adverse events, and device-related serious and unanticipated adverse events. Prespecified descriptive end points included time in open vs closed-loop systems; the percentage of sensor glucose values below, within, and above target range (71-180 mg/dL), including at night time; changes in HbA1c, insulin requirements and body weight; and measures of glycemic variability. End points were collected during both periods and analyzed with SAS(SAS Institute), version 9.4.
Results
Of the 124 participants (mean age, 37.8 years [SD, 16.5]; men, 44.4%), mean diabetes duration was 21.7 years, mean total daily insulin dose was 47.5 U/d (SD, 22.7), and mean HbA1c was 7.4% (SD, 0.9). Over 12 389 patient-days, no episodes of severe hypoglycemia or ketoacidosis were observed. There were 28 device-related adverse events that were resolved at home. There were 4 serious adverse events (appendicitis, bacterial arthritis, worsening rheumatoid arthritis, Clostridium difficile diarrhea) and 117 adverse events not related to the system, including 7 episodes of severe hyperglycemia due to intercurrent illness or other nonsystem causes.
The system was in closed-loop mode for a median of 87.2% of the study period (interquartile range, 75.0%-91.7%). Glycated hemoglobin levels changed from 7.4% (SD, 0.9) at baseline to 6.9% (SD, 0.6) at study end . From baseline to the end of the study, daily dose of insulin changed from 47.5 U/d to 50.9 U/d, and weight changed from 76.9 kg to 77.6 kg. The percentage of sensor glucose values within the target range changed from 66.7% at baseline to 72.2% at study end. Sensor and reference glucose values collected during the hotel stays were in good agreement, with an overall mean absolute relative difference of 10.3% (SD, 9.0).
Discussion
To our knowledge, this is the largest outpatient study to date and it demonstrated that hybrid closed-loop automated insulin delivery was associated with few serious or device-related adverse events in patients with type 1 diabetes. Limitations include lack of a control group, restriction to relatively healthy and well-controlled patients, the relatively short duration, and an imbalance between the length of the study periods. Differences in HbA1c levels may be attributable to participation in the study. A similar study in children is under way. Longer-term registry data and randomized studies are needed to further characterize the safety and efficacy of the hybrid closed-loop system.
[su_button url=”http://jama.jamanetwork.com/article.aspx?articleid=2552454″ target=”blank” style=”flat” background=”#64b243″ size=”6″ center=”yes” radius=”5″ icon=”icon: angle-right”]Continue Reading[/su_button]