Month: April 2016
-

Islet Transplantation May Correct Type 1 Diabetes, Study says
Original article written by Stephen Feller and published by United Press International on April 26, 2016. Click here to read the original article. WASHINGTON, April 18 (UPI) — Transplants of islet cells, […]
-

Salk Makes Step Toward Cellular Diabetes Treatment
Original article written by Bradley J. Fikes and published by The San Diego Union-Tribune on April 15, 2016. Click here to read the original article. Salk Institute scientists say they’ve discovered […]
-
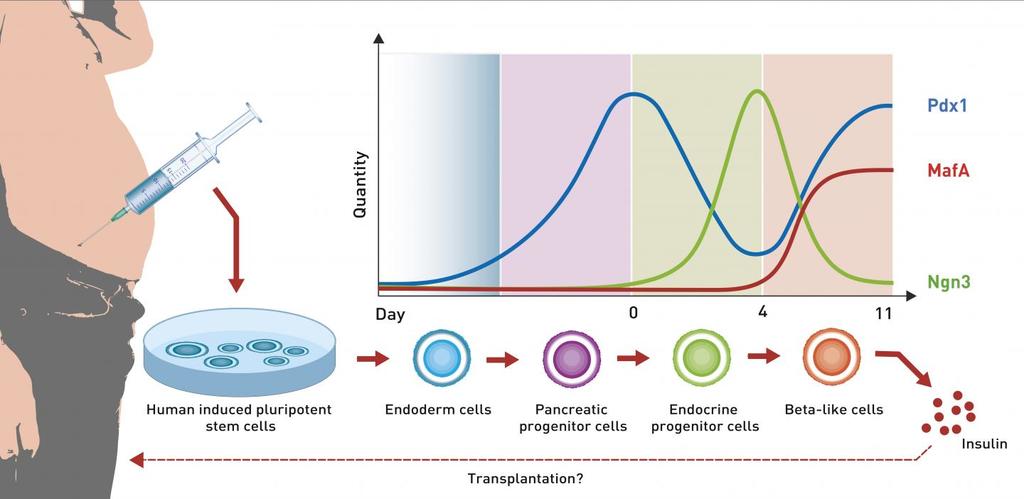
Beta Cells From Love Handles
Original article written by Eth Zurich and published by EurekAlert! American Association for the Advancement of Science (AAAS) on April 11, 2016. Click here to read the original article. Researchers led […]
-

Consequences and Burdens of Type 1 Diabetes in Low-Income and Middle-Income Countries
Diabetes is a global epidemic. Just consider the following from the World Health Organization’s fact sheet on diabetes: -In 2014 the global prevalence of diabetes was estimated to be 9% […]
-

How to Get Involved in the Global Fight Against Diabetes
The Diabetes Research Connection’s mission is to connect donors with early-career scientists, enabling them to perform peer-reviewed, novel research designed to prevent and cure type 1 diabetes, minimize its complications […]
-

The Bionic Pancreas Is Getting Closer To Reality
Original article published in TIME HEALTH by Alexandra Sifferlin on April 1, 2016. To view the original article Click Here. The invention could seriously change the management of type-1 diabetes The […]
