Month: October 2014
-
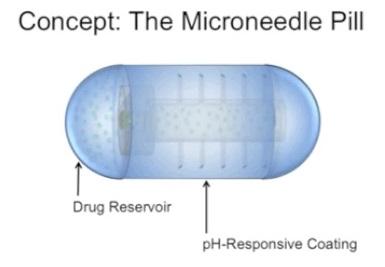
Mad Scientists and Their Diabetes Breakthroughs
Imagine popping a pill covered in tiny steel needles – that you cannot in any way feel – to quickly and effectively lower your…
-
Type 1 Diabetes Discovery: Stem Cells Make Millions of Human Insulin Cells
As we are all aware, type 1 diabetes is an autoimmune disease where the body destroys insulin-producing beta cells…
-

Is Diabetes All in Your Head?
Researchers at Mount Sinai have identified a key mechanism behind diabetes that may start in the brain. Set of molecules found to…
-
Long Acting Insulin Versus Immediate Acting Insulin for Type 1 Diabetes Patients Compared in Review
For many people with type 1 diabetes, daily treatment and management of the condition is a big part of their life. But is…
-

A New Approach to Diabetes Discovery
Welcome to the Diabetes Research Connection, the first nonprofit to use crowd funding to enable innovative diabetes investigations…
-

Novel Diabetes Research
The Diabetes Research Connection is pleased to present THREE extraordinary research projects. Scientists from the…
