Month: October 2020
-

Connect For A Cure: November 2020 Newsletter
DRC has distributed over $400,000 to research projects like Dr. Hughes’s and Dr. Racine’s in 2020 alone! We have received three times the average amount of applications for funding of […]
-

Understanding the Relationship between Diabetes and COVID-19
COVID-19 is a relatively new virus, and one that researchers are continuing to learn more about every day. Studies have shown that individuals with underlying health conditions are at increased […]
-

Could Type 1 Diabetes be an Effect of COVID-19?
As the coronavirus pandemic continues on, researchers are learning more about the wide range of effects that it has on individuals. The disease presents differently in different people, ranging from […]
-
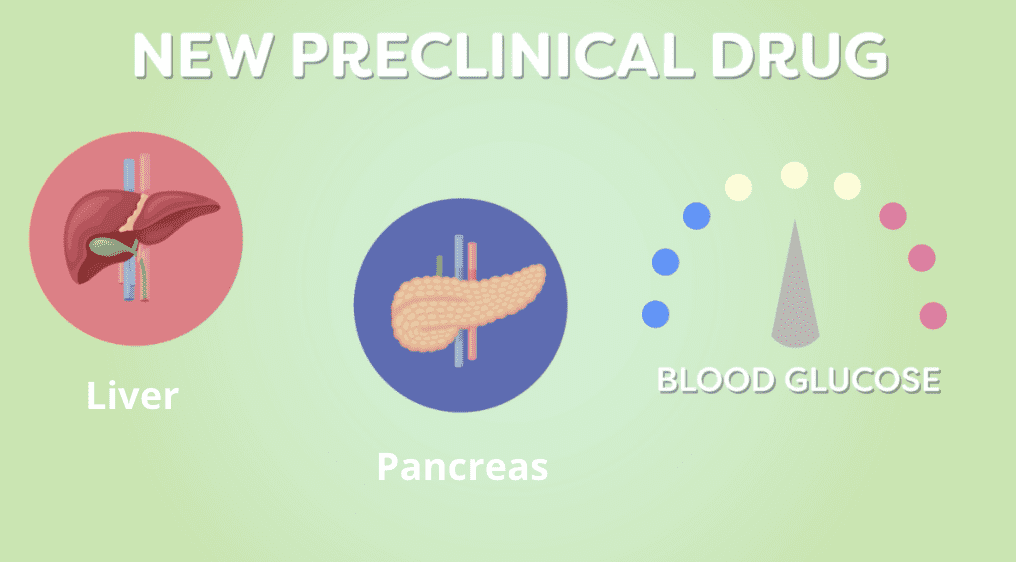
A New Approach to Treating Diabetes and Its Effects
For decades, researchers have been studying cellular changes in the body that contribute to the development of diabetes. They have created a wide array of treatment options to help manage […]
-

Exploring the Role of Metabolic Memory in Diabetes Complications
As the immune system slowly destroys insulin-producing pancreatic beta cells, a hallmark sign of type 1 diabetes, the body has an increasingly difficult time controlling blood glucose levels. These cells […]
-

Digging Deeper into the Role of Genetics in Type 1 Diabetes
Type 1 diabetes is a complex disease. While researchers know what it does to the body, they are still unclear on exactly why this happen and what triggers this response. […]
-
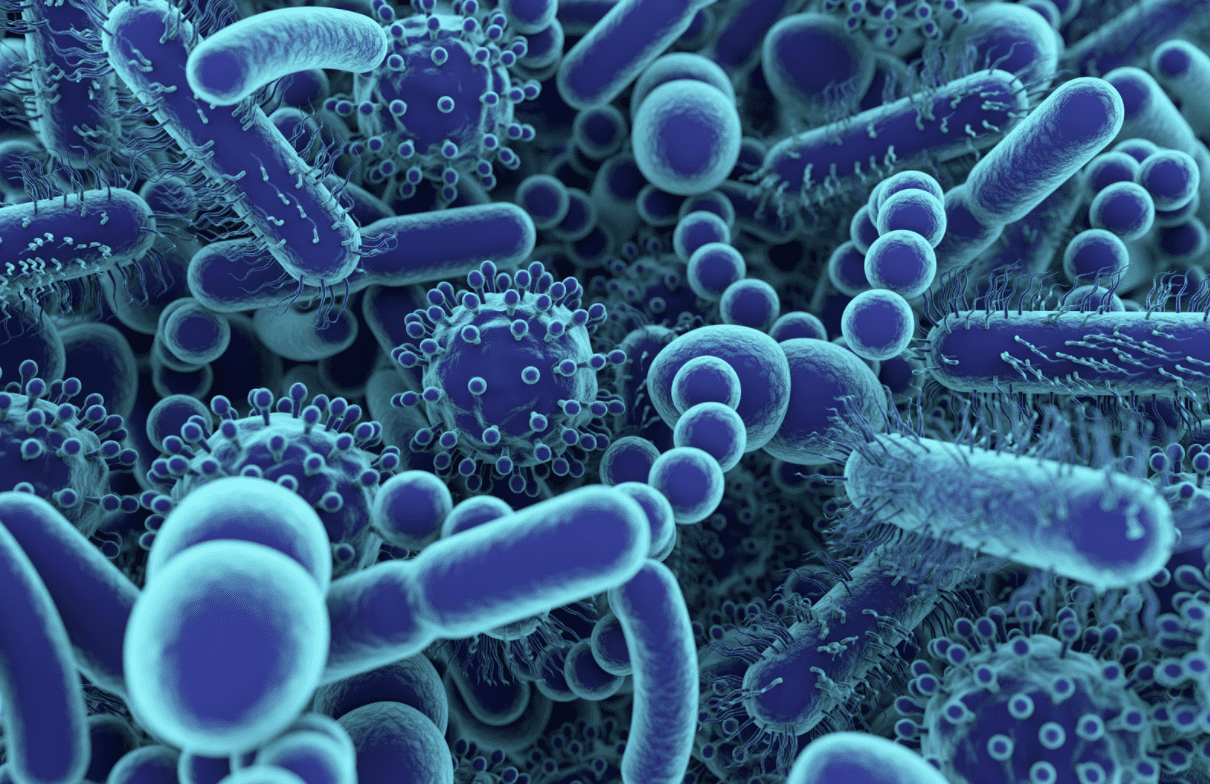
Examining Gut Microbiome Differences
The composition of gut bacteria – both good and bad – differs in everyone. Each person has their own makeup dependent upon diet, environment, geographical location, and other factors. In […]
-
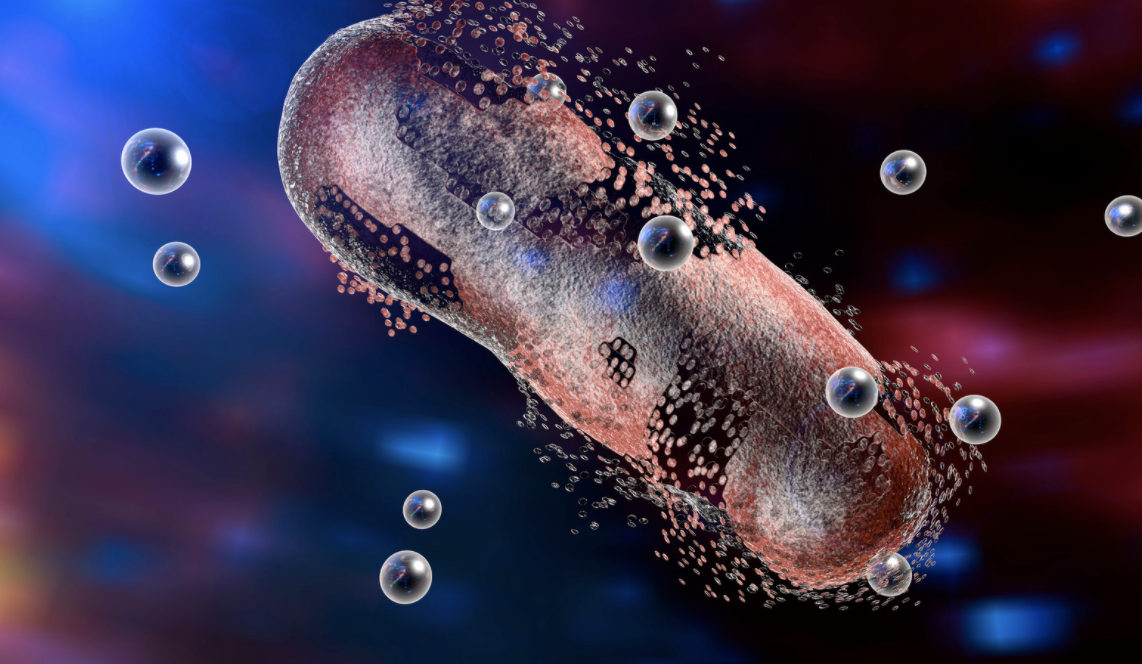
Leveraging Nanoparticles in Diagnosing and Treating Type 1 Diabetes
Leveraging Nanoparticles in Diagnosing and Treating Type 1 Diabetes Medical technology has seen significant advancements over the years helping to improve healthcare in many ways. An area of recent focus […]
-
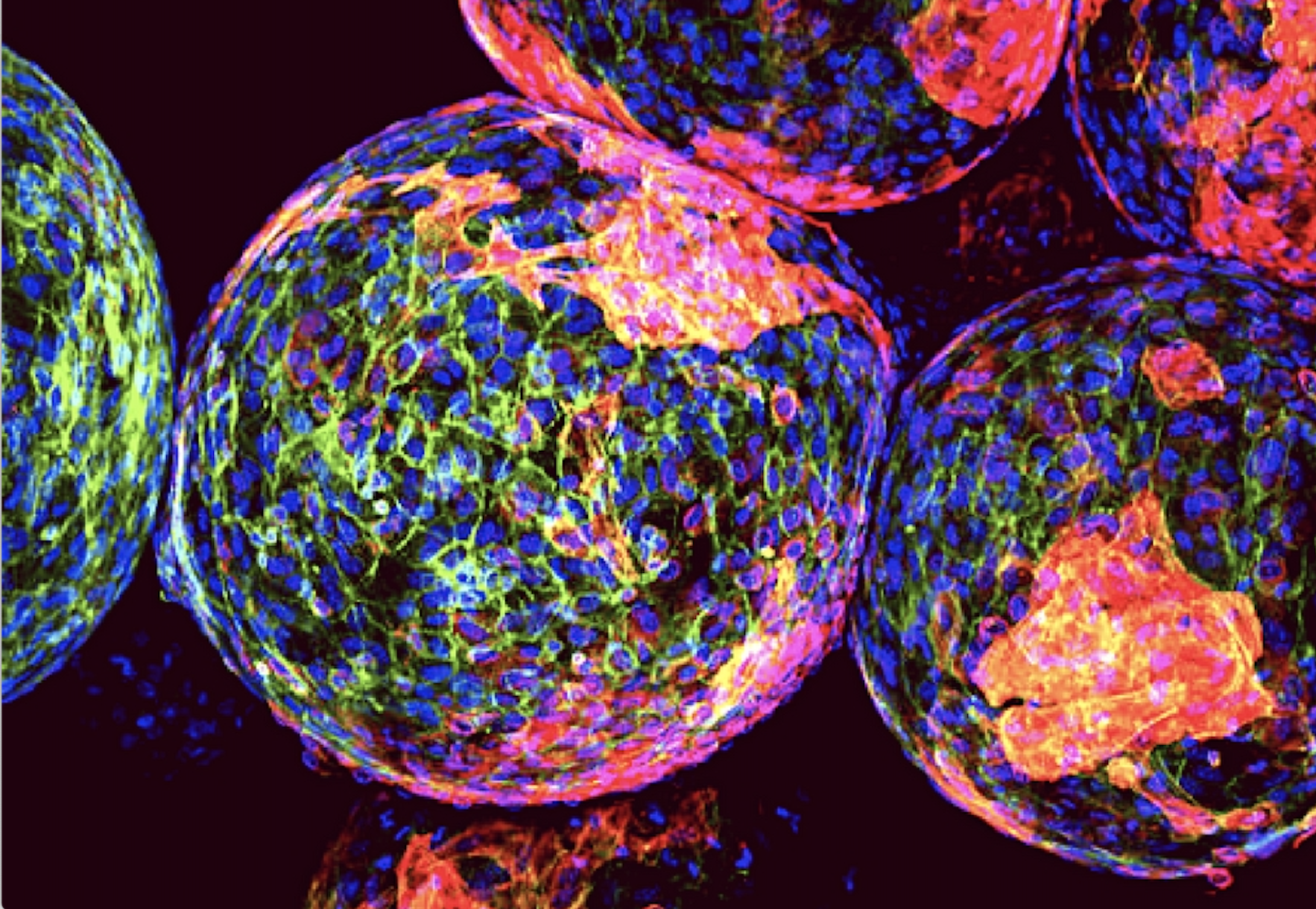
Enhancing Protection of Insulin-Producing Beta-Cells
Enhancing Protection of Insulin-Producing Beta-Cells Insulin-producing beta-cells play a critical role in managing blood sugar by automatically releasing insulin in response to increased blood glucose levels. In individuals with type […]
-

Detecting Diabetic Retinopathy Using Artificial Intelligence
Detecting Diabetic Retinopathy Using Artificial Intelligence Managing blood sugar is not the only challenge that individuals with type 1 diabetes (T1D) face. There can be numerous complications that arise from […]
-
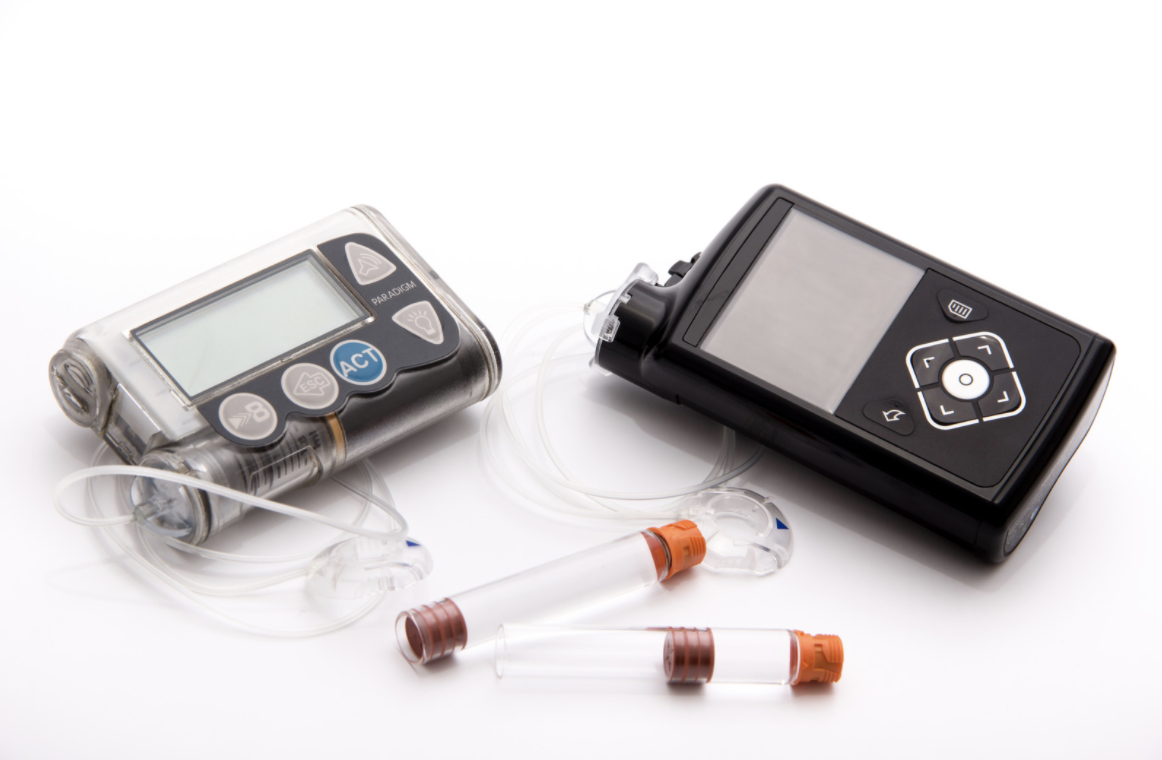
Enhancing Quality of Life and Time-in-Range Through Automated Insulin Delivery Systems
Automated Insulin Delivery Systems Type 1 diabetes (T1D) is a condition that must be managed 24 hours a day, seven days a week, 365 days a year. Even with careful […]
-
Speeding Up Insulin Activation for Managing Type 1 Diabetes
Speeding Up Insulin Activation for Managing Type 1 Diabetes Individuals with type 1 diabetes (T1D) rely on daily insulin injections to effectively manage blood sugar levels and maintain glycemic control. […]
