Month: March 2016
-
KUSI News anchor Brad Perry interviews Duc Dong, Ph.D. and Joseph Lancman, Ph.D
KUSI News anchor Brad Perry interviews Duc Dong, Ph.D. and Joseph Lancman, Ph.D of Sanford Burnham Prebys Medical Discovery Institute in La Jolla on ground breaking research regarding type 1 diabetes. […]
-

KUSI Good Morning San Diego Interviews Dr. Steven Ruderman
The Background of Dr. Steven Ruderman Professional Highlights Dr. Steven Ruderman is an illustrious figure in the world of medicine, notably in the field of Diabetes. Throughout his career, Ruderman […]
-
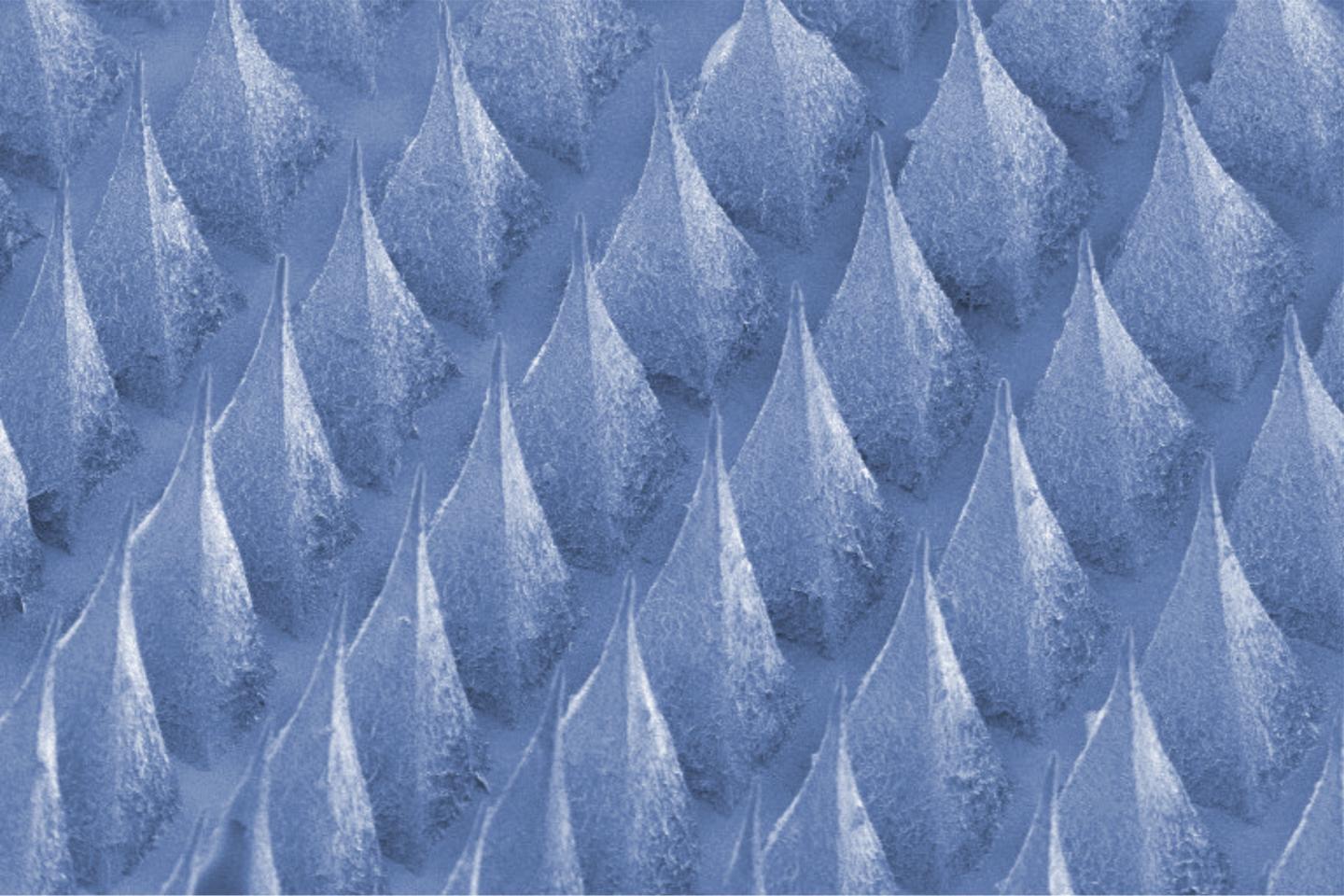
Scientists Create Painless Patch Of Insulin-Producing Beta Cells To Control Diabetes
Original article published by EurekAlert! American Association for the Advancement of Science (AAAS) on March 14, 2016. Click here to read the original article. This new ‘smart cell patch’ developed at UNC […]
-
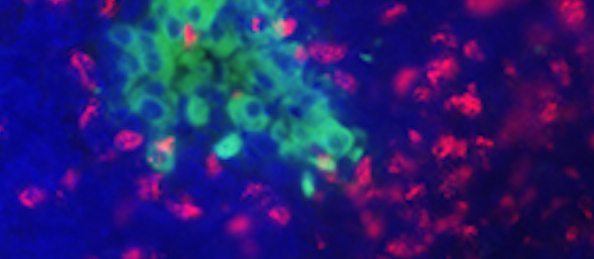
The Next Step in Preventing Diabetes
Original article written by German Research For Environmental Health on March 15, 2016. Click here to read the original article. Neuherberg, March 15, 2016. A team of scientists at Helmholtz Zentrum […]
-

Simplified Autologous Hematopoietic Stem Cell Transplantation Shows Promise In Type 1 Diabetes
Original article written by Cantú-Rodríguez OG, et al. J Clin Endocrinol Metab. 2016;doi:10.1210/jc.2015-2776 on February 23, 2016. Click here to read the original article. In patients with type 1 diabetes, simplified […]
