Month: July 2015
-

FDA Approves The Second Phase Of Dr. Denise Faustman’s Clinical Testing Of A Type 1 Diabetes Vaccine
After nearly 20 years of research, Massachusetts General Hospital researcher Denise Faustman, M.D., Ph.D., has made a promising advance in her quest to cure type 1 diabetes.
-
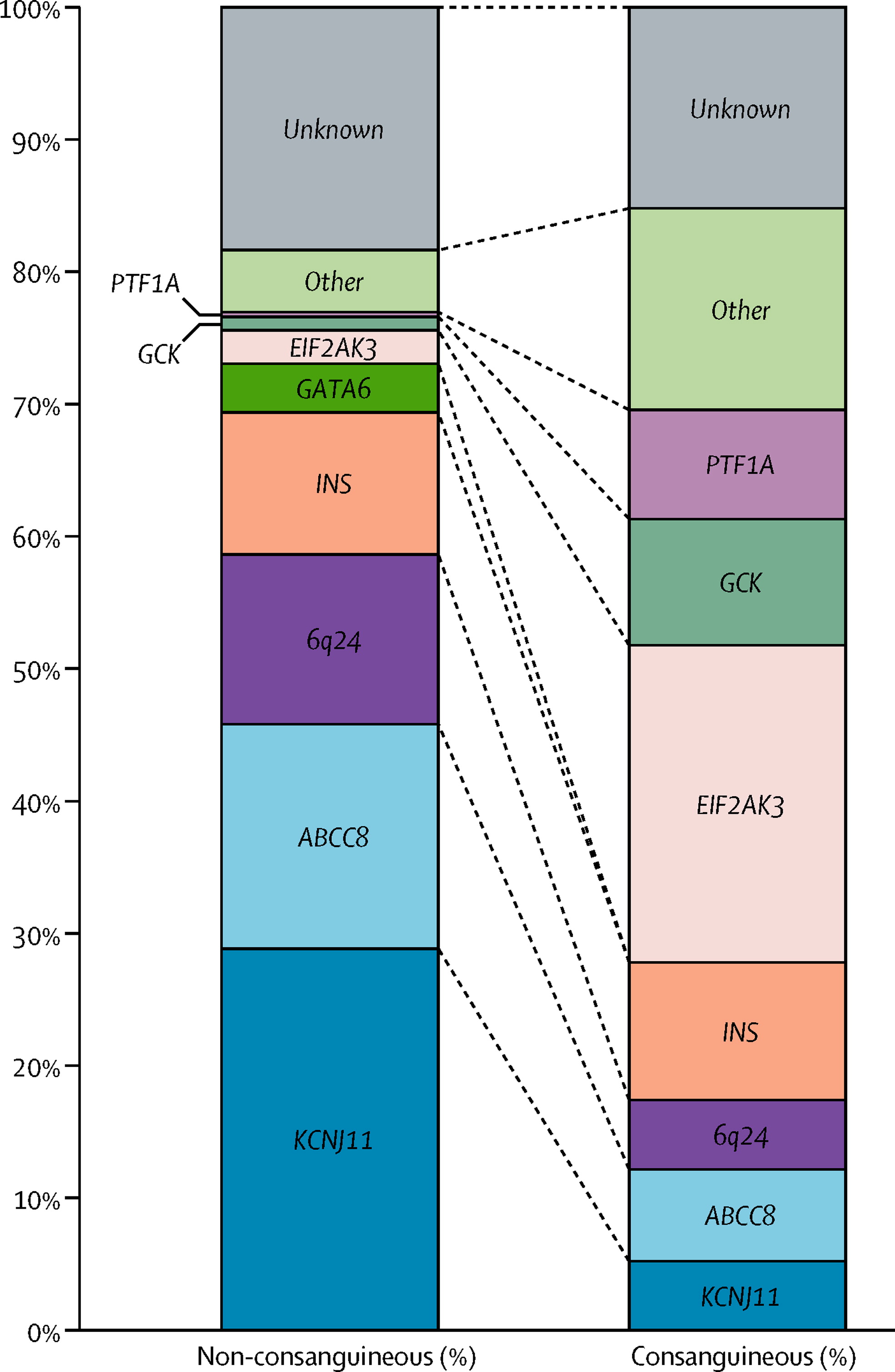
The Effect Of Early, Comprehensive Genomic Testing On Clinical Care In Neonatal Diabetes
Traditional genetic testing focuses on analysis of one or a few genes according to clinical features; this approach is changing as improved sequencing methods enable simultaneous analysis of several genes.
-
Psoriasis Drug May Help Preserve Pancreas Cells In Type 1 Diabetes
Psoriasis Drug May Help Preserve Pancreas Cells in Type 1 Diabetes Used early after diagnosis, drug appears to have long-lasting effects, study reports
-

Caring Causes: Diabetes Research Connection
One out of every hundred Americans has type 1 diabetes. Millions of children and adults struggle with this autoimmune disease.
-

Young People With Type 1 Increasingly Likely To Be Obese, Experts Urge Dietary Changes
Young people with type 1 diabetes are increasingly likely to be obese, according to new research.
-
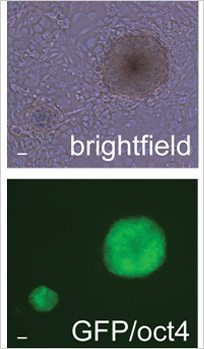
Production of iPS Cells: Discovery of the 5th Element
Reprogramming an already specialised cell into a stem cell is a scientific feat coveted by many researchers.
-

New Group Seeks to Jumpstart Diabetes Research
The Diabetes Research Connection (DRC) is a new organization that helps fresh-on-the-scene scientists conduct type 1 research.
