Month: June 2015
-

Halting Diabetes: UCSF Researchers Publish Study
This week, novel findings by UCSF researchers for developing therapeutic strategies to combat diabetes…
-

Crowdfunding Platform Created To Fund Type 1 Diabetes Research
DRC connects donors directly with early-career scientists, enabling them to perform peer-reviewed research…
-
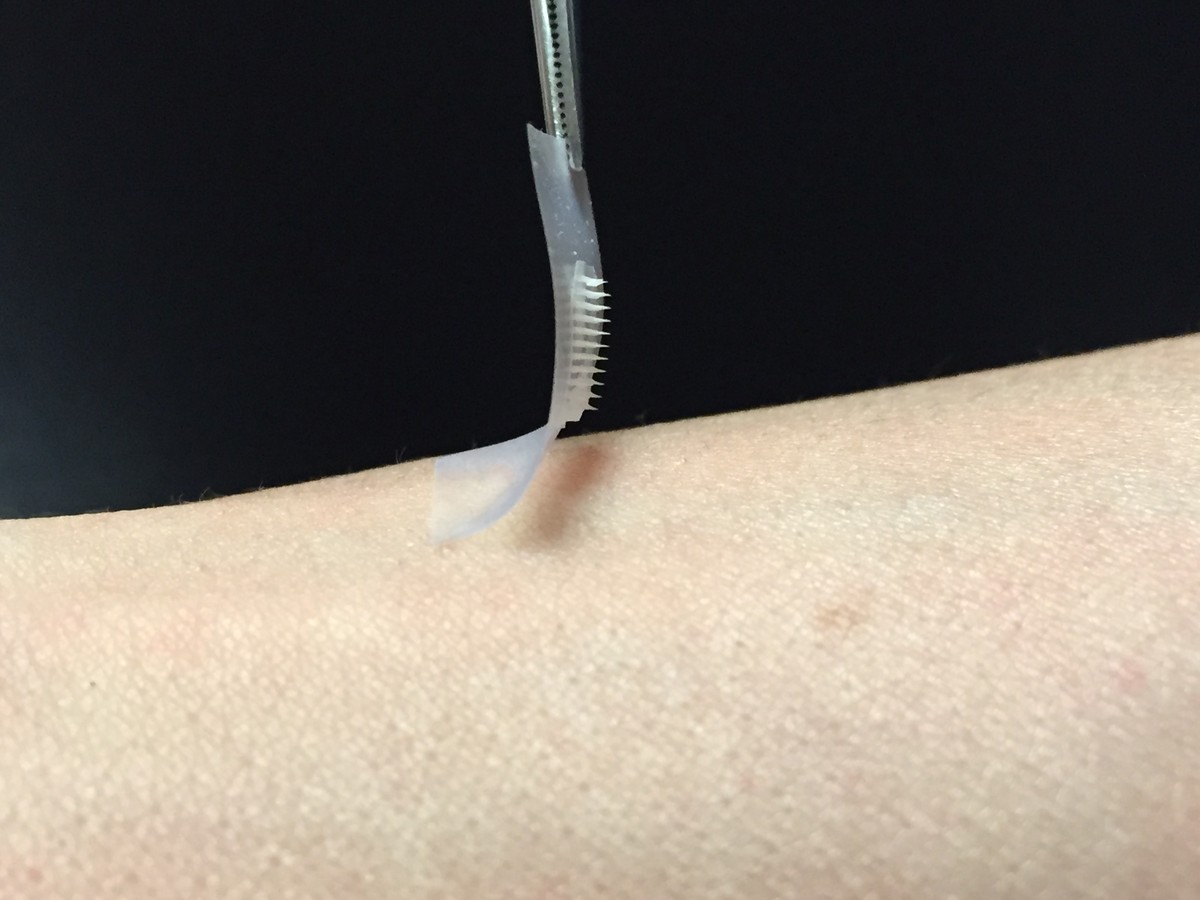
The Smart Insulin Patch: Replace Injections For Diabetic Patients?
Researchers in North Carolina are developing a “smart” insulin patch…
-
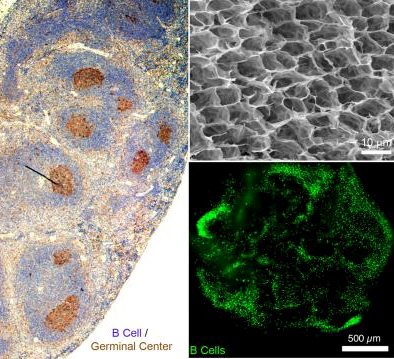
First Working Synthetic Immune Organ With Controllable Antibodies
Promises to lead to better understanding of the immune system, develop new therapies, improve testing of new classes of…
-

Gene Sheds Light on Development of Type 1 Diabetes, Autoimmune Disease
Type 1 diabetes and multiple sclerosis are types of autoimmune disease – where the immune…
