Month: December 2014
-
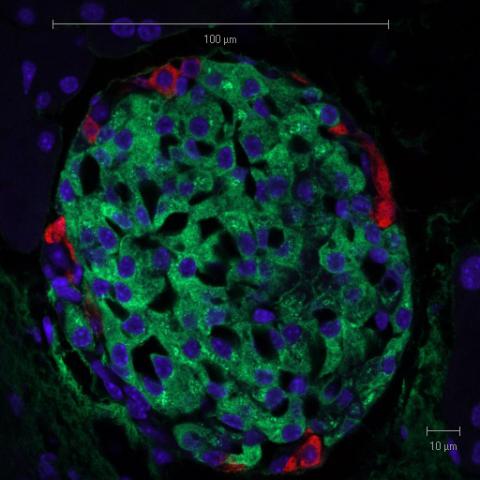
Islet Cell Transplantation After Pancreas Removal May Help Preserve Normal Blood Sugar
Surgery to remove all or part of the pancreas and then transplant a patient’s own insulin-producing islet cells appears to be a safe…
-
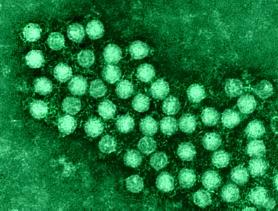
Detection of Enterovirus Infection in Insulin Producing Cells in Patients Newly Diagnosed with Type 1 Diabetes
Norwegian scientists with European partners have found evidence for the presence of Enterovirus in pancreatic islets…
-

Novel Type 1 Diabetes Treatment Shown to Work on Human Beta Cells Transplanted Into Mice
A chemical produced in the pancreas that prevented and even reversed type 1 diabetes in mice had the same effect on…
